In June of this year, Professor Stephen Bradforth of USC and his collaborators made the cover of Science magazine with their stunning results on how liquid ammonia transforms into a liquid metal as more alkali metals are dissolved into it. We are thrilled to have Professor Bradforth speak to our section about these exciting results this coming Sunday, November 8, 2020. Due to the pandemic, this lecture will be conducted over Zoom, and the details are below. Please join us for an exceptional talk on truly outstanding results. The abstract of the talk appears below.
Speaker: Professor Stephen Bradforth
Title: Probing the transition from solution electrolyte to liquid metal
Date: Sunday November 6, 2020
Time: 3:00 PM
Zoom Location: Please us the “Contact” link above to request the Zoom link for this lecture.
Cost: Free
Please use the following links download
The abstract
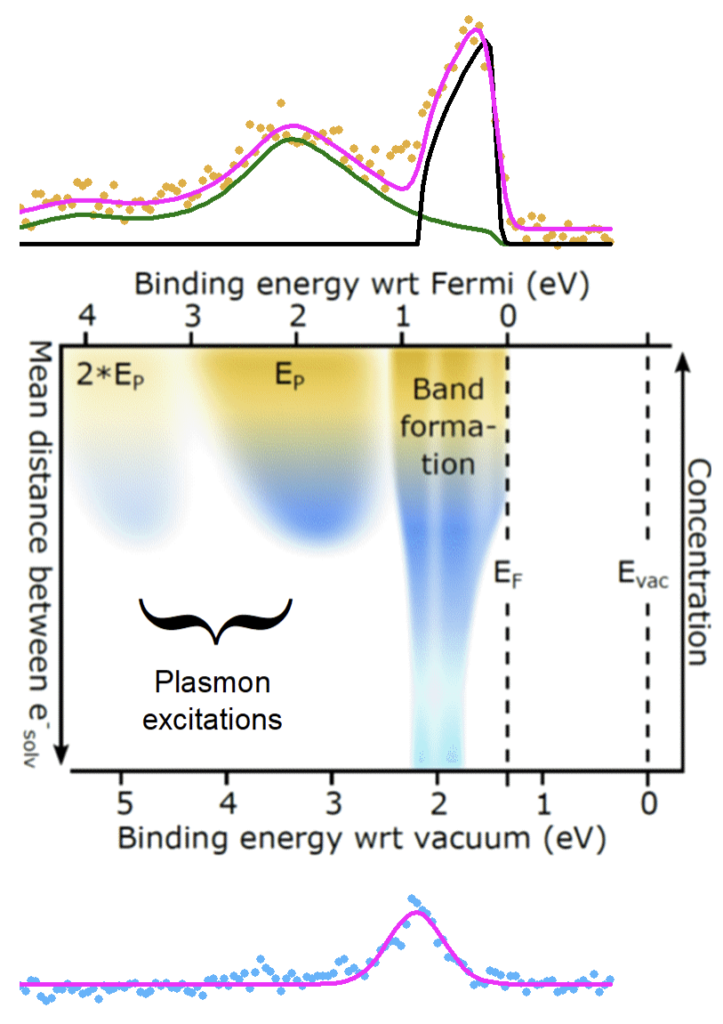
Few metals have the same allure as a clean surface of liquid mercury. Another liquid metal, metallic liquid ammonia, can built from the ‘ground up’ starting from an electronically insulating hydrogen-bonded liquid very similar to water. This system has been known since the early 1800’s and is frequently used as an organic reagent, but here we use it to explore what nanoscale phenomena cause metallic behavior. At low concentrations of a dissolved alkali metal like lithium, dilute solvated electrons (and alkali cations) are formed which give the electrolyte solution a characteristic blue color. As the concentration is increased, the solution first turns inky black and then develops a bronze metallic sheen. When blue, the excess solvated electrons exist as localized entities in the gaps between ammonia molecules; as the number of excess electrons is increased, electrons combine to form di-electrons (two electrons spin pair inside a solvent cavity) and then finally become fully percolated throughout the hydrogen bonded liquid – a delocalized sea that changes ammonia into a metal.
Our research used synchrotron X-ray light to eject electrons from microjets of the liquid solutions to discover how bound electrons are bound within the solvent. The photoelectron spectra map out how the electron binding-to-the-solvent changes from dilute localized species (bottom, blue) to delocalized metallic species (top, bronze).
 Stephen Bradforth earned his PhD at the University of California, Berkeley, and carried out postdoctoral research at the University of Chicago. He joined the Department of Chemistry at the University of Southern California (USC) in 1996. He has served as department chair and is currently USC Dornsife Divisional Dean for Physical Sciences and Mathematics. As a physical chemist, his lab designs experiments to gain a deeper understanding of how the inter-connected motions of molecules impact chemical reactions in complex but frequently encountered environments – such as the aqueous milieu of cells or in functional molecular materials. His research applies ultrafast laser techniques to address contemporary scientific challenges from solar energy conversion, damage to DNA and cancer nanomedicine. In education, he has been active in recent national efforts, spearheaded by the Research Corporation for Scientific Advancement and the Association of American Universities, to reform undergraduate STEM education in research-intensive universities.
Stephen Bradforth earned his PhD at the University of California, Berkeley, and carried out postdoctoral research at the University of Chicago. He joined the Department of Chemistry at the University of Southern California (USC) in 1996. He has served as department chair and is currently USC Dornsife Divisional Dean for Physical Sciences and Mathematics. As a physical chemist, his lab designs experiments to gain a deeper understanding of how the inter-connected motions of molecules impact chemical reactions in complex but frequently encountered environments – such as the aqueous milieu of cells or in functional molecular materials. His research applies ultrafast laser techniques to address contemporary scientific challenges from solar energy conversion, damage to DNA and cancer nanomedicine. In education, he has been active in recent national efforts, spearheaded by the Research Corporation for Scientific Advancement and the Association of American Universities, to reform undergraduate STEM education in research-intensive universities.
His honors include David and Lucile Packard Fellowship in Science and Engineering, a Cottrell Scholarship from the Research Corporation for Scientific Advancement; he is a Fellow of the American Physical Society, the American Association for the Advancement of Science and of the Royal Society of Chemistry.
